
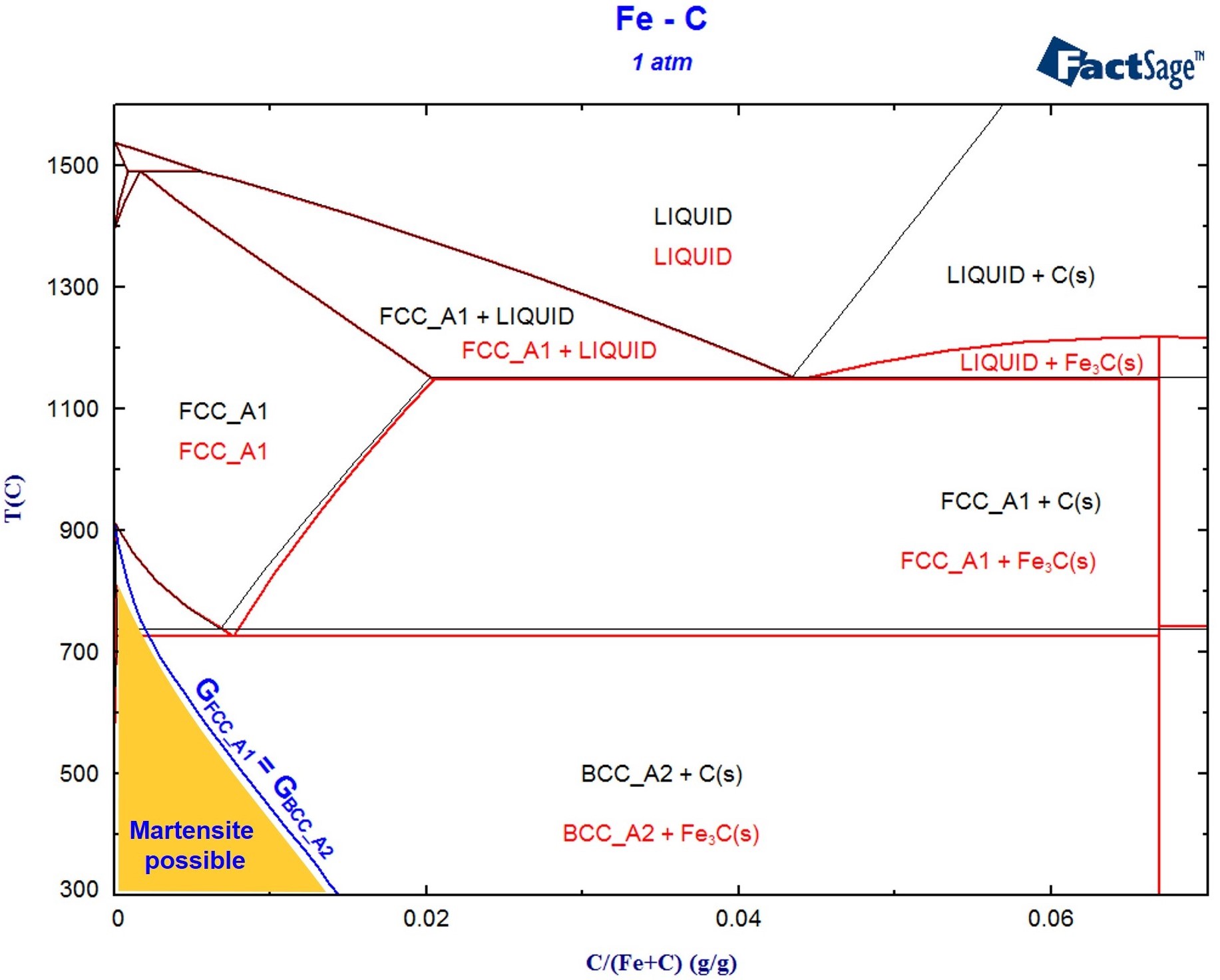
The position of the eutectic equilibrium point is found to shift toward a low-melting-point component by 5-10%, and the eutectic point temperature is lightly overestimated in comparison with the experimental results and the FactSage data. The discrepancies between the calculated and published data become more noticeable for systems containing larger and more polarizable cations. The error in describing the liquidus lines in the phase diagrams of the fluoride mixtures with the use of the tabulated ionic radii and polarizabilities is at most 15% as compared to the available FactSage data. The liquidus lines in the binary phase diagrams LiF-NaF, LiF-KF, NaF-KF, NaF-CsF, and KF-CsF of the eutectic type are calculated. This is shown in the following phase diagram in red as an overlay of the.
performing calculations with FactSage software. A thermodynamic perturbation theory model is developed to take into account the additional charge-dipole contribution to the free energy of molten alkali halide systems, and it is used to describe the melt-crystal equilibria in binary salt mixtures with common anions. The Aqueous Phase Diagram is a new type of phase diagram in FactSage 7.1 that we have developed for displaying phases in equilibrium with the aqueous phase. It is a common misconception that thermodynamic calculations can only. thermodynamics (and the idea the energy is minimized at equilibrium) in order to.


 0 kommentar(er)
0 kommentar(er)
